Allgenesis Presented Three Posters at ARVO 2019
Allgenesis Receives Notice of Acceptance of Australian Patent for Fusion Protein of VEGF Trap and Disintegrin
2019-02-13Phase 2a SURPH Trial for AG-86893
2019-05-24May 4th, 2019
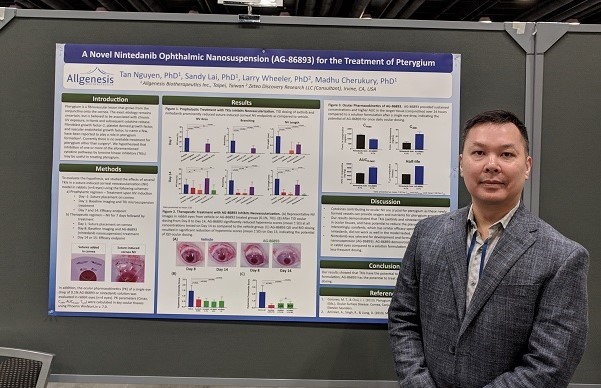
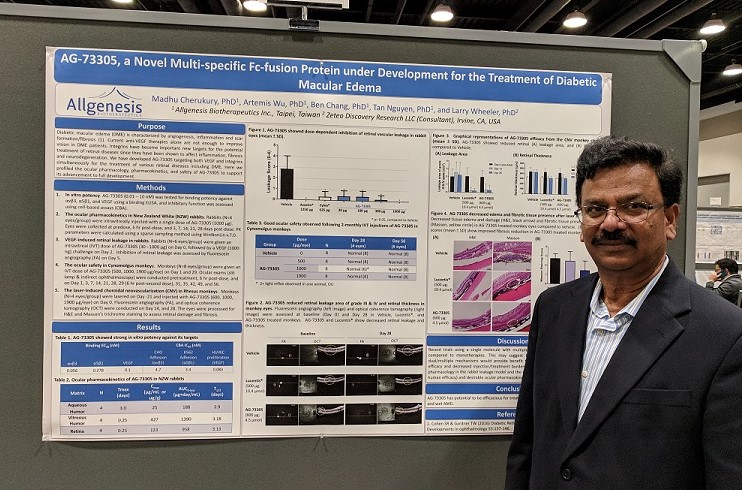
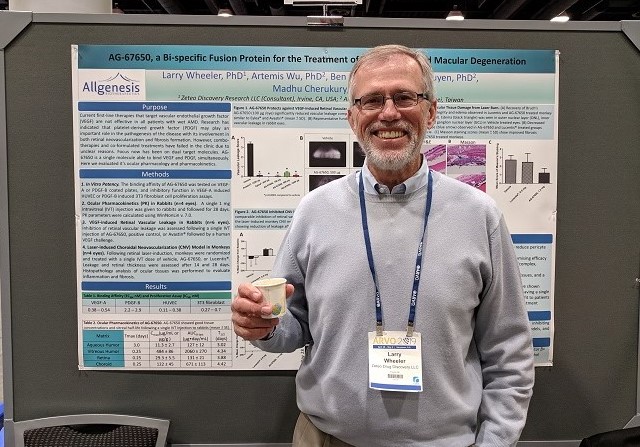
Allgenesis attended the 2019 ARVO Conference (Association for Research in Vision and Ophthalmology) that was held from April 26th and May 3rd in Vancouver, B.C. Canada. Three abstracts from Allgenesis were submitted for AG-86893, AG-73305, and AG-67650, and all were accepted to be presented in the daily Poster Sessions. Tan Nguyen, PhD, presented on AG-86893, Madhu Cherukury, PhD, presented on AG-73305, and Larry Wheeler, PhD, presented on AG-67650. Our posters were well received at the conference, garnering questions and interest from academia and industry experts. Allgenesis hopes to build upon the favorable reception at the conference to develop potential licensing or collaborative opportunities.

About Allgenesis
Allgenesis is a clinical stage biopharmaceutical company based in Taipei, Taiwan. The company is focused on research and development of novel medicines for the treatment of eye diseases. Current projects in the pipeline include AG-73305, a potential blockbuster drug for the treatment of DME, wAMD, and other retinal diseases, AG-86893 for pterygium, and AG-67650 for wAMD.
About AG-86893
AG-86893 is a topical ocular eye drop reformulation of a marketed oral drug, a multi-tyrosine kinase inhibitor targeting receptors of growth factors such as VEGF, PDGF and FGF. AG-86893 is being developed for the treatment of pterygium, following a 505(B)(2) pathway. Currently, there is no approved treatment for pterygium other than surgery. A clinical Phase 2a study (trial name: SURPH*) for AG-86893 is being conducted for pterygium patients in Australia with FPE in 4Q2018.
*SURPH = Study of the Response to AG-86893 in patients with Pterygium Hyperemia
About AG-73305
AG-73305 is a first-in-class molecule specifically designed for the treatment of DME, wAMD, and other retinal diseases. AG-73305 is a single fusion protein that simultaneously binds to VEGF and integrins with high potency and specificity. During the discovery stage, AG-73305
demonstrated promising efficacy in protecting the blood-retina-barrier in a rabbit POC model and in a laser-induced CNV monkey model. AG-73305 has a desirable ocular pharmacokinetic profile and was well-tolerated in monkeys after intravitreal injection.
About AG-67650
AG-67650 is a first-in-class, single molecule specifically designed for treating wAMD. AG-67650 simultaneously binds to VEGF and PDGF with high potency and specificity. AG-67650 demonstrated promising efficacy in protecting the blood-retina-barrier in a rabbit POC model and in a laser-induced CNV monkey model. AG-67650 has a desirable ocular pharmacokinetic profile. Currently, Allgenesis is looking for collaboration opportunities for AG-67650 for wAMD and related indications.
