AG-80308
Overview
AG-80308, in-licensed from Allergan (acquired by Abbvie in May 2020) for use in ophthalmology, is a topical ocular eye drop being developed for the treatment of Dry Eye Disease. AG-80308 has a novel first-in-class mechanism of action to reduce inflammation in dry eye disease by directing towards resolution pathways. Its novel mechanism of action presents a unique opportunity for a well differentiated therapeutic for this significant disease.
AG-80308 has anti-inflammatory activity in animal models and has the potential to reduce inflammation on the ocular surface of patients with dry eye disease. It presents a novel pathway to potentially treat dry eye patients. A Phase 1 clinical study was completed with AG-80308 in healthy and dry eye patients to assess the safety, tolerability, and pharmacokinetics and was found to be safe. Further studies on the dose response of the drug will be the next steps for Allgenesis to further assess its safety and efficacy in dry eye patients.
Disease profile
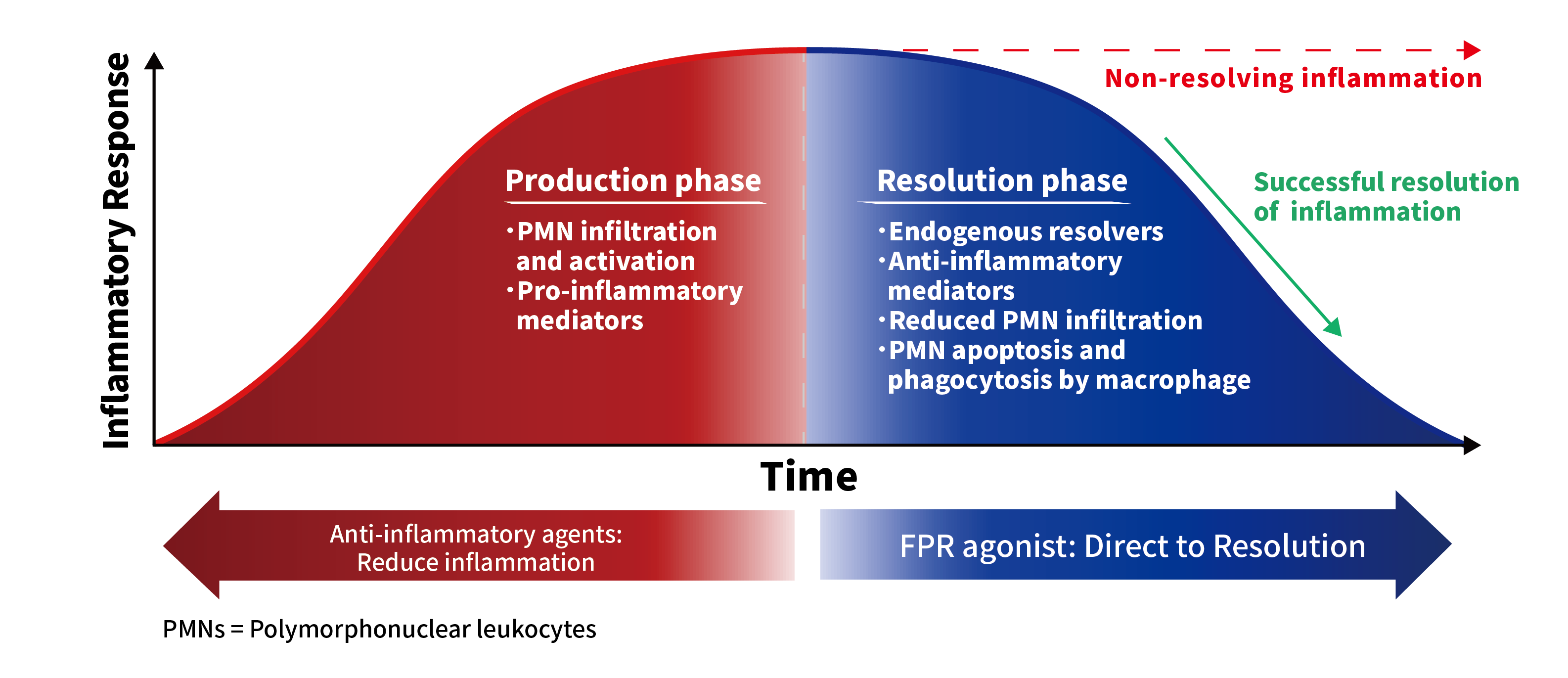
Mechanism of Action
AG-80308 would be a First-in-Class, formyl peptide receptor (FPR) agonist formulated as an aqueous solution eye drop. Unlike immunosuppressants and steroids, which act to reduce pro-inflammatory mediators, AG-80308 acts to promote resolution of inflammation by activating anti-inflammatory mediators and immune cells.